√ダウンロード heterogeneous mixture vs homogeneous mixture examples 753046-Heterogeneous mixture vs homogeneous mixture examples
A homogeneous solution tends to be identical, no matter how you sample it Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures A heterogeneous mixture has two or more phases and the components can be individually identified A homogeneous mixture is uniform; A mixture is basically a combination in which two or more substances are combined A solution is mainly of two types that are homogeneous mixtures and heterogeneous mixtures A solution is a homogeneous mixture of solute and a solvent A solute is a substance that gets dissolved in a solvent whereas the solvent is the substance that dissolves a

Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture
Heterogeneous mixture vs homogeneous mixture examples
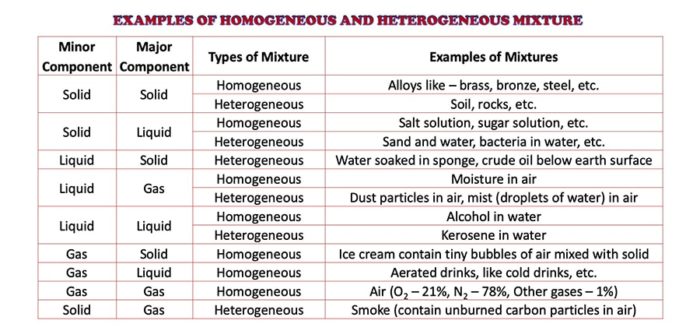
Heterogeneous mixture vs homogeneous mixture examples- Some common examples of the mixture in our daily life are – air, milk, fruit juice, medicines, honey, tap water, brass, bronze, etc Air is a mixture of oxygen, nitrogen, carbon dioxide gases It also contains water vapor, dust particles, and traces of inert gases Mixtures are of two types – homogeneous and heterogeneous mixtureA chemical mixture combines two substances that maintain their own properties when combined Heterogeneous mixtures are made up of a nonuniform composition, while homogeneous mixtures are made up of a uniform compositionFor example, water and sand is a heterogeneous mixture — you can easily separate the sand from the water




The Homogeneous And Heterogeneous Mixture Diagram Quizlet
As a rule, it is conceivable to physically isolate parts of a heterogeneous blend, however not a homogeneous blend For instance, you can expel oat from milk and pasta from sauce On the off chance that you are uncertain about whether a blend is homogeneous or heterogeneous, consider its example estimate Pure substances in a heterogeneous mixture can be easily separated without using such processes The homogeneous mixture is only in one phase of matter The heterogeneous mixture is always in two or more than two different phases of matter When we mix alcohol in water, it exists in a uniform physical stateHeterogeneous mixture 1 It has a nonuniform composition 2 Example of Heterogeneous mixture sea water, concrete, a mixture of salt water and sand The most common homogeneous mixture examples Heterogeneous Mixture;
A homogeneous mixture An alloy is a solid composition of two or more pure metals or nonHomogeneous vs heterogeneous The difference between homogeneous mixtures and heterogeneous mixtures is a matter of scale the heterogeneous mixture can be seen on beaches where sand included many particles like coral, shells and organic matter, etc they all can be separated easily hence known as a heterogeneous mixture but when we take a large amount of sand, it's impossible to separate all the matter, which terns as a homogeneous mixture example Examples of a heterogeneous mixture Colloid A colloid is an example of a heterogeneous mixture where the components exist in two distinct phases;
Homogeneous vs Heterogeneous Mixtures Mixtures can be either heterogeneous or homogeneous Unlike a heterogeneous mixture, a homogeneous mixture is a mixture that is uniform throughout;• Apple juice is homogeneous • Orange juice with pulp is heterogeneous • Chocolate dough is homogeneous • Italian salad dressing is heterogeneous Homogenous vs Heterogeneous Matter created by Simmy8 on 21 Jun 12, enabled by jimmy Sciences Medium level (75% of success) 15 questions 52 033 players Classify the following substances and mixtures as either homogeneous or heterogeneous 1




10 Examples Of Mixtures
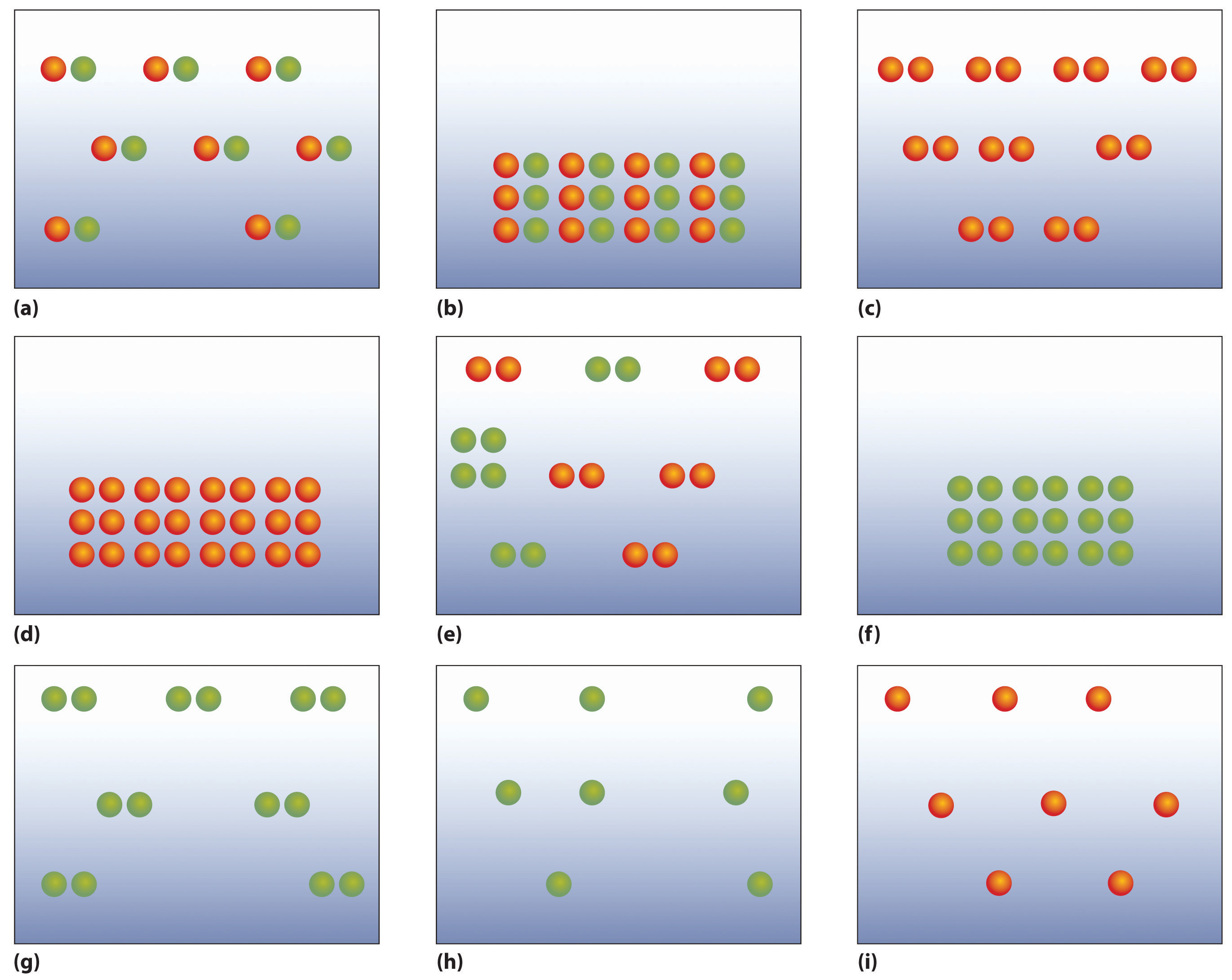



Chapter 1 3 A Description Of Matter Chemistry Libretexts
#Homogeneous #Heterogeneous #Mixture #Difference #homogeneous #heterogeneous #mixture chemistry,heterogeneous,homogeneous,homogeneous mixture example,heterogeneous mixture examples,homogeneous vs heterogeneous matter,types of heterogeneous mixture,homogeneous solution,homogeneous and heterogeneous mixtures,homogeneous mixtures,heterogeneous Salt and water, for example, are homogeneous mixtures, as is sugar plus water As described by the dictionary of Chemistry, a heterogeneous mixture is a combination in which the constitution is not regular and smooth The elements are not homogeneous in their composition The components can't be dissolved readily Examples of homogeneous mixtures include Salty water — a mixture of salt and water Ruby — a mixture of Al 2 O 3 and Cr 2 O 3 Gasoline — a mixture of various hydrocarbons Brass — a mixture of Cu and Zn Air without clouds — a mixture of various gases Heterogeneous mixtures contain two or more components that can be seen, which can




Chemistry For Kids Chemical Mixtures




Homogeneous Mixture Definition Examples Tutors Com
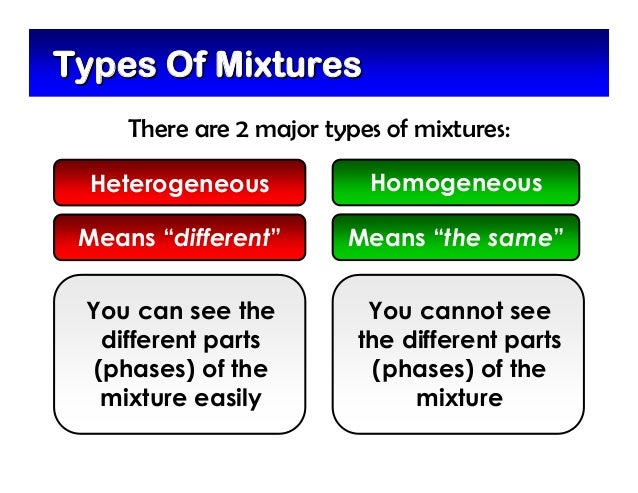
The Difference Between Heterogeneous and Homogeneous Mixtures Homogeneous Mixture Examples You can't pick out components of a homogeneous mixture or use simple mechanical means to Heterogeneous Mixture Examples Heterogeneous mixtures are more common than homogeneous mixtures Usually,Q Type of mixture that DOESN'T HAVE the same composition in every partA heterogeneous mixture is simply any mixture that is not uniform in composition — it's a nonuniform mixture of smaller constituent parts By contrast, a mixture that is uniform in composition is a homogenous mixtureFor the purposes of this discussion, "not uniform" means anything that clearly has different parts visible to the naked eye or that could be easily separated from each
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures



Homogeneous Heterogeneous Mixture Definition Examples Selftution
Summary A mixture is a physical blend of two or more components, each of which retains its own identity and properties in the mixture A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture All solutions would be considered homogeneous A heterogeneous mixture is a mixture in which the composition is not uniform throughout theThis means that the different components of the mixture cannot be distinguished from one another in some wayHomogeneous mixtures 10 Heterogeneous and Homogeneous Mixtures A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout There is only one phase of matter observed in a homogeneous mixture at a time




What Is A Heterogeneous Mixture Definition And Examples




This Presentation Discusses Homogeneous And Heterogeneous Mixtures Provides Examples Explains How A Particle Di Heterogeneous Mixture Pure Products Mixtures
41/5 (136 Views 11 Votes) Heterogeneous products are products with attributes that are significantly different from each other, which makes it difficult to substitute one product for another An example of a heterogeneous product is a computer Commodities are generally a good example of homogeneous products See full answer Mixtures are formed when two or more compounds or elements combine together without participating in a chemical change Each component in a mixture retains its own chemical properties and makeup Scientists recognize two types of mixtures homogeneous and heterogeneous The latter is a mixture with a nonuniform compositionA homogeneous mixture is a composition of two or more pure substances in a definite proportion, displaying uniform characteristics These mixtures do not exhibit a sharp melting point since they melt at different temperatures Some Natural Examples of Homogeneous Mixtures Sources of Water




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Pure Substances Elements Compounds Homogenous Heterogenous Mixture Examples And Problems Youtube
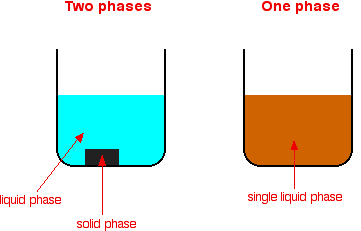
By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase Properties of homogeneous mixtures It has the same uniform appearance The particles in this mixture are very small and cannot be seen easily The mixture is dissolved completely Only one state of matter is observed in this mixture at a time Salt and water solution is an example of this mixtureDiverse salad dressing, chex mix, rocks, salad, chicken noodle soup Homogeneous mixture vs Heterogeneous mixture




Pure Substances And Mixtures Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture
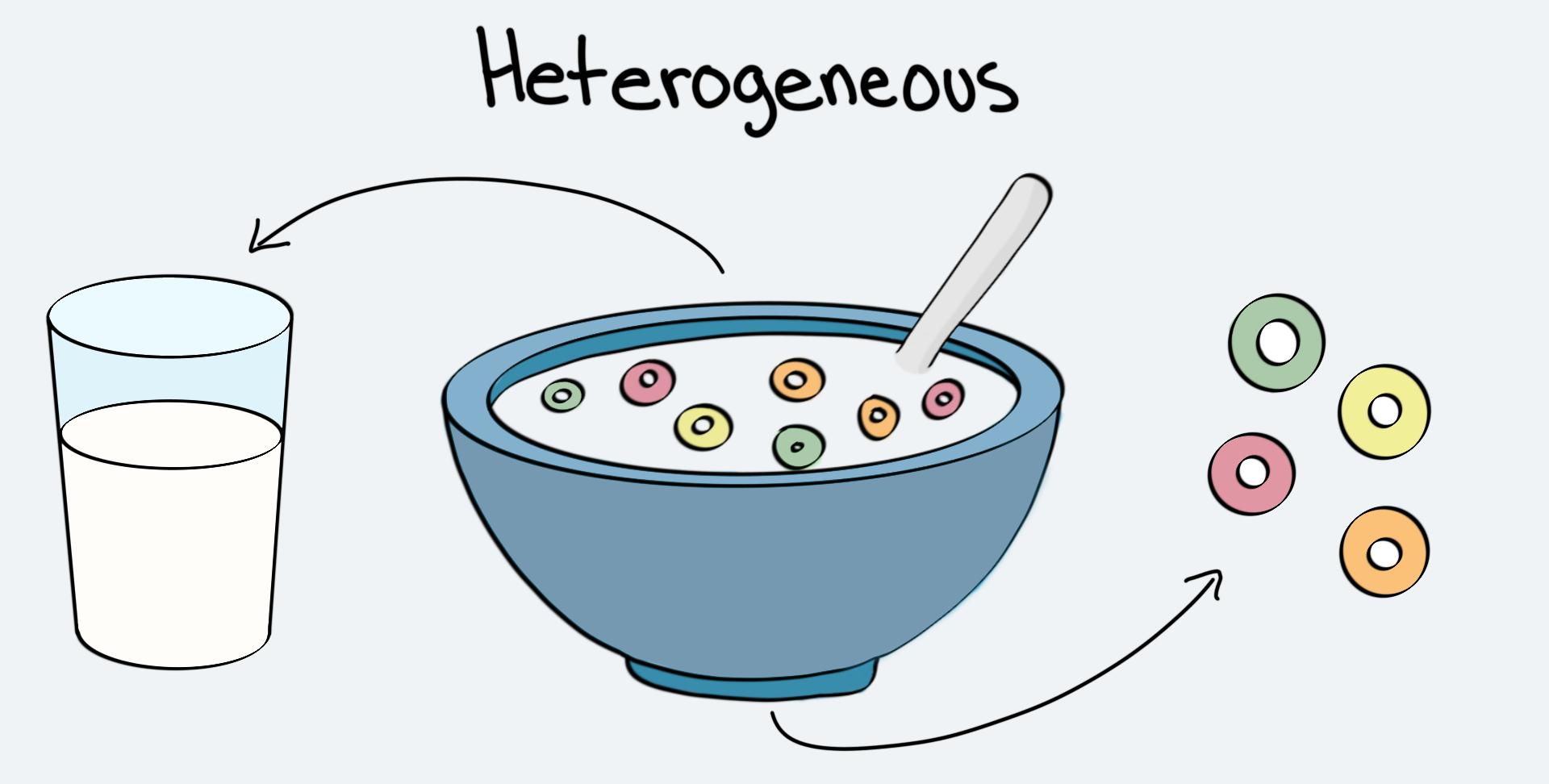



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
Q If they are not the same throughout, they are called answer choices Homogeneous Types and examples of homogeneous mixtures Liquid mixture Pure water, vinegar, coconut oil, etc Gas Mixtures Air in the Atmosphere Solid mixtures For example mineral ores, alloys such as steel, bronze, brass What is a Heterogeneous mixture? Unlike homogeneous mixtures, heterogeneous mixtures do not have the same composition throughout Oil and water is a fine example of a heterogeneous mixture When oil is mixed with water, the separation of two layers is visible to the eye The following picture shows this phenomenon clearly
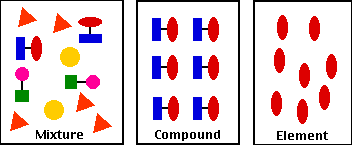



Mixtures And Compounds




Mixture Wikiwand
Therefore, the individual components cannot be separately identified When allowed to stay undisturbed, the components of a homogeneous mixture do not settle down Solutions and colloids are the two main categoriesStart studying Heterogeneous vs Homogeneous Mixtures Learn vocabulary, terms, and more with flashcards, games, and other study tools The pure substances in a heterogeneous mixture can be easily separated without using such processes Phase of Matter The homogeneous mixture is only in the one phase of matter The heterogeneous mixture is always in two or more than two different phases of matter Examples When we mix alcohol in water, it exists in the uniform physical state




Pure Substances And Mixtures Unit 2 Solution Solvent
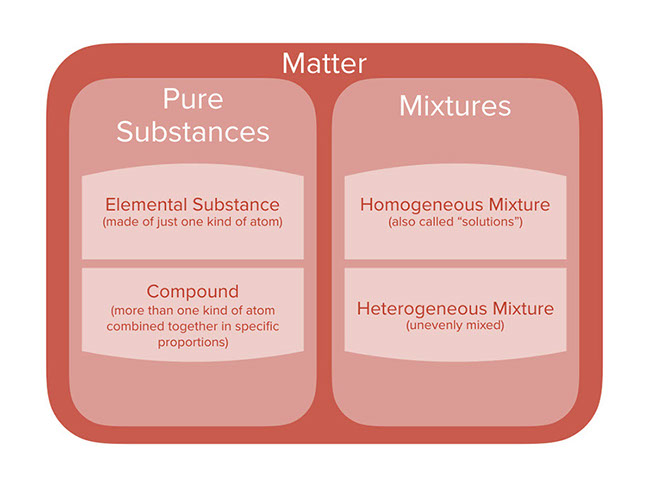



Lesson Categories Of Chemicals And Mixtures
A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample Conversely, a heterogeneous mixture has components whose proportions vary throughout the sample"Homogeneous" and "heterogeneous" are not absolute terms but depend on context and the size of the sample In chemistry, a homogeneousHeterogeneous mixtures are composed of two or more substances that exhibit specific characteristicsThis chemistry video tutorial explains the difference between homogeneous and heterogeneous mixtures within the subtopic of the classification of matter It




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




1 Differentiate Between Homogon Eous Aid Heterogeneous Mixt Scholr
In this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference betweenDispersed phase and continuous phase The insoluble particles of a colloid do not settle down completely as the particles are small in size, usually ranging between 107 to 103 cm The components in a heterogeneous mixture can easily be seen with a naked eye, but for better results, you can utilize a microscope Examples Homogeneous Mixture Some examples of homogeneous mixtures are most alloys, seawater, brass, vinegar, air, blood, natural gas, etc Heterogeneous Mixture Salt and pepper together make a heterogeneous



Sci8u1l2




Mr Chapman Chemistry 30 What Is A Solution A Solution Is A Mixture Or A Combination Of Two Or More Things A Solution Is Also Known As A Homogeneous Ppt Download
For example, the physical eye can pick up the substances that make up this type of mixture because they are large enough to be seen Like homogeneous mixtures, examples of heterogeneous mixtures can include solids, liquids, and gases Some liquid examples include salad dressing and red wine vinegar A gas example can include air with clouds inWell, it is important to note that in a heterogeneous mixture, the components are seen in two layers or phases So, talking about water, it is a homogeneous mixture in itself because water comprises of elements like nitrogen, oxygen, and other gaseous substances Homogeneous mixtures and heterogeneous mixtures are different from one anotherHeterogeneous Mixture Examples A heterogeneous mixture is a mixture that, quite simply, is not uniform throughout Hetero means different You could separate the parts of the mixture from each otherthis is one of the hallmarks of a heterogeneous mixtureAnother characteristic is that the different substances that make up a heterogeneous mixture keep their own properties




Heterogeneous And Homogeneous Mixtures Cut And Paste Sorting Activity
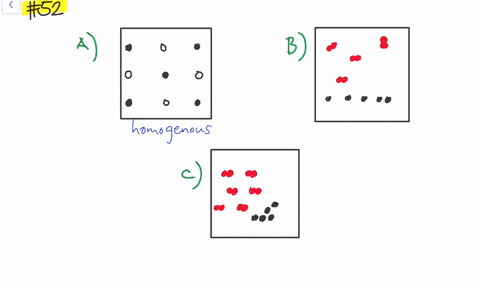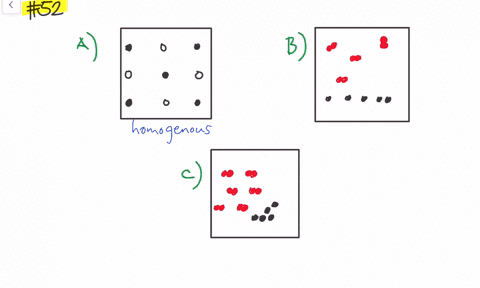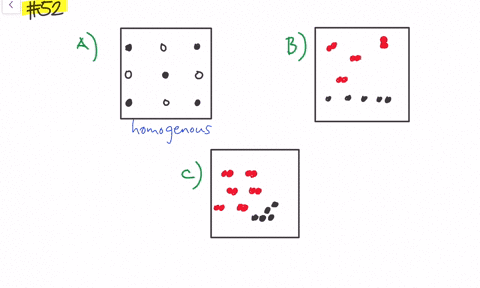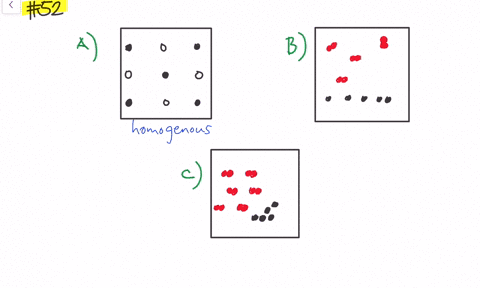



Solved Give Three Examples Each Of Heterogeneous Mixtures And Homogeneous Mixtures
Other examples are white vinegar and corn oil What sets these mixtures apart from heterogeneous mixtures is the particle size of the mixed constituents The very air we breathe is a homogeneous mixture while the milk you drink, with floating marshmallows, is a heterogeneous mixtureHomogeneous mixture Heterogeneous mixture 1) These are called as solutions These are called as suspensions/colloids 2) Substances are Uniformly distributed These substances are Unevenly distributed 3) These are not visible to the naked eye, but visible through the microscopeActivity This one page worksheet will give your students practice with heterogeneous and homogeneous mixtures The topics of the questions include prefix meanings, examples, differences between the two types of mixtures, and the definition of mixtures The worksheet involves critical thinking skills and will




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Homogeneous Mixture Definition Examples Tutors Com
Examples of heterogeneous mixtures include The mixture of sand and water The mixture of oil and water Petroleum or Crude oil and so on Please read on the Fractional Distillation of Petroleum here Concrete is an heterogeneous mixture comprising of cement, sand, gravel and water Blood is an heterogeneous mixture of plasma and cellsHomogeneous mixtures exist in one phase of matter at a time You will not see liquid water and solid water together in a homogeneous mixture That means your glass of ice water, with ice cubes floating in it, is a heterogeneous mixture of homogeneous mixtures Homogeneous mixtures cannot be expressed as chemical formulasA homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid, and solid




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards
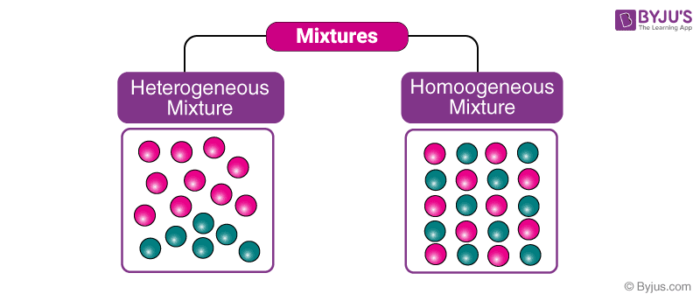



Heterogeneous And Homogeneous Mixture Differences Videos Examples
Examples of heterogeneous mixtures Two types of heterogeneous mixtures a physical combination of two or more substances composed of unlike parts; A mixture consists of sugar in water is a homogeneous mixture because we can't see particles of sugar in the water, as they are dissolved thoroughly A mixture consisting of oil in water is an example of the heterogeneous mixture as the oil cannot be mixed in the water and we can easily see them I hope you like my post about "Difference Heterogeneous and Homogeneous Mixtures In the lesson, you learned about heterogeneous mixtures and homogeneous mixtures Another name for a homogeneous mixture is a solution




Homogeneous And Heterogeneous Mixtures Youtube



1
Even a mixture of oil and water is heterogeneous because the density of water and oil is different, which prevents uniform distribution in the mixture Examples of homogeneous mixtures are milkshakes, blended vegetable juice, sugar dissolved in coffee, alcohol in water, and alloys like steel Even the air that's in our atmosphere is a homogeneous mixture of various gases




Examples Of Heterogeneous Mixtures Types Made Simple



Heterogeneous Vs Homogeneous Solutions Chapter 12 Solutions




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets
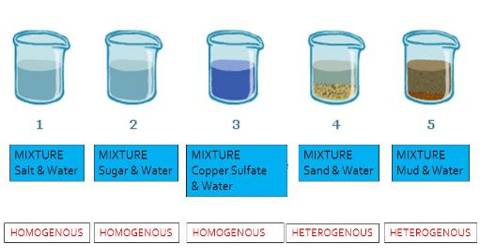



Homogeneous Mixture Experiment Qs Study
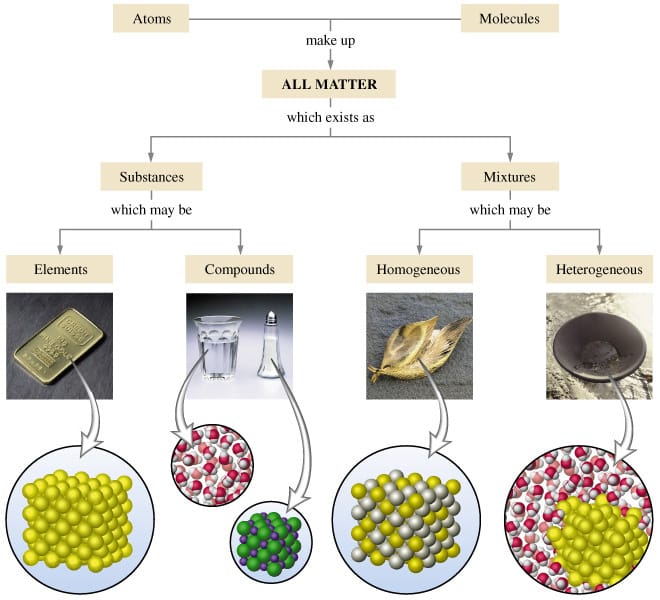



Classifying Matter Schoolworkhelper
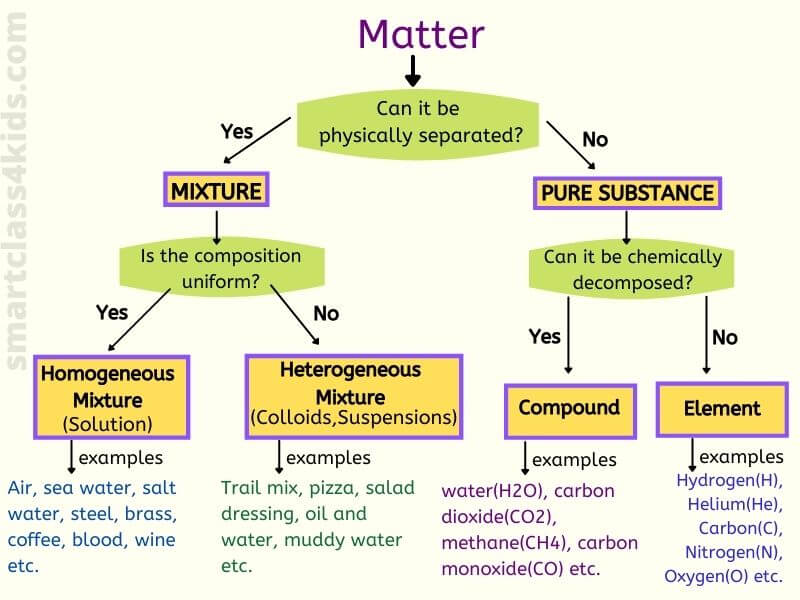



What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




Homogeneous Or Heterogeneous Mixtures Practice Worksheet




Differentiate B W Homogeneous And Heterogeneous Mixtures Teachoo



Mixture




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




Classify Each Substance As An Element A Compound A Homogeneous Mixture Or A Heterogeneous Mixture Brainly Com
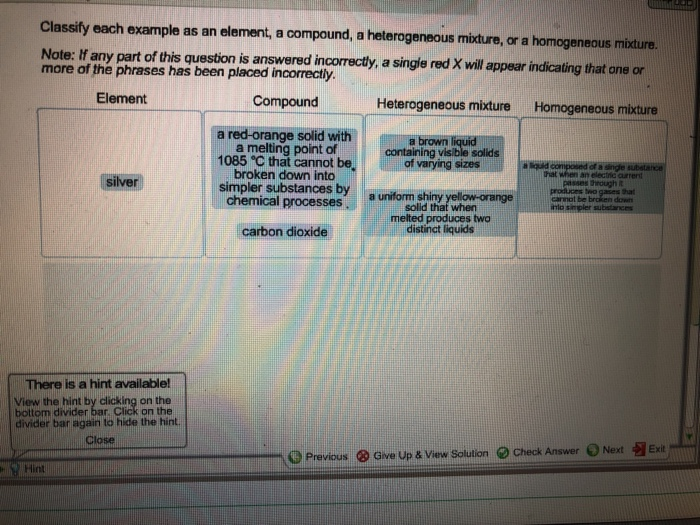



Classify Each Example As An Element A Compound A Chegg Com



Types Of Catalysis



Http Sites Isdschools Org Grade6 Remote Learning Resources Useruploads 05 11 Science6 Schimmelsmartwynn May13 Pdf



Homogeneous And Heterogeneous Mixtures




Solutions And Mixtures Flashcards Quizlet




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




What Is A Homogeneous Mixture Definition And Examples



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Explain The Difference Between A Homogeneous And Heterogeneous Mixture Give An Example For Each Brainly Com




Pure Substances And Mixtures 1 Pure And Mixed




Classify Mixtures As Homogeneous Or Heterogeneous Worksheet




Homogeneous And Heterogeneous Mixtures Activities



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Classification Of Matter Chemistrygod



Mixture



Www Austincc Edu Mohan Documents 04 Worked Examples Pdf




Homogeneous And Heterogeneous Mixture Heterogeneous Mixture Homogeneous Mixture Mixtures




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Mixture




Homogeneous Mixture Definition Examples Tutors Com




Compound Vs Mixture Difference And Comparison Diffen



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Module 1 Hierarchy Of Matter And Separation Methods Ppt Download




Heterogeneous And Homogeneous Mixtures With Examples Study Guide Brighthub Education



Q Tbn And9gcsbe Ybqypf3mkoozl7w6krqjqo5iirs Nvzkdphtkiwz1wjxbm Usqp Cau




Mixture Homogeneous And Heterogeneous Mixtures Ck 12 Foundation




Examples Of Homogeneous Mixtures Solid Liquid And Gas




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
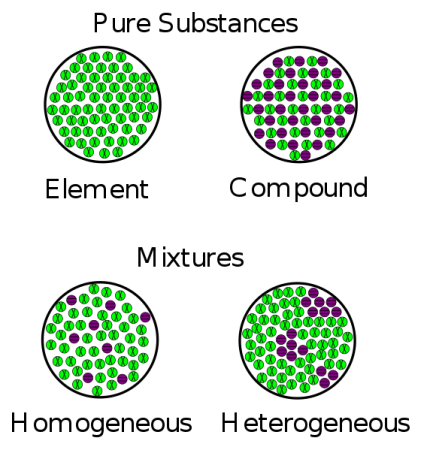



Heterogeneous Mixture Definition Science Trends
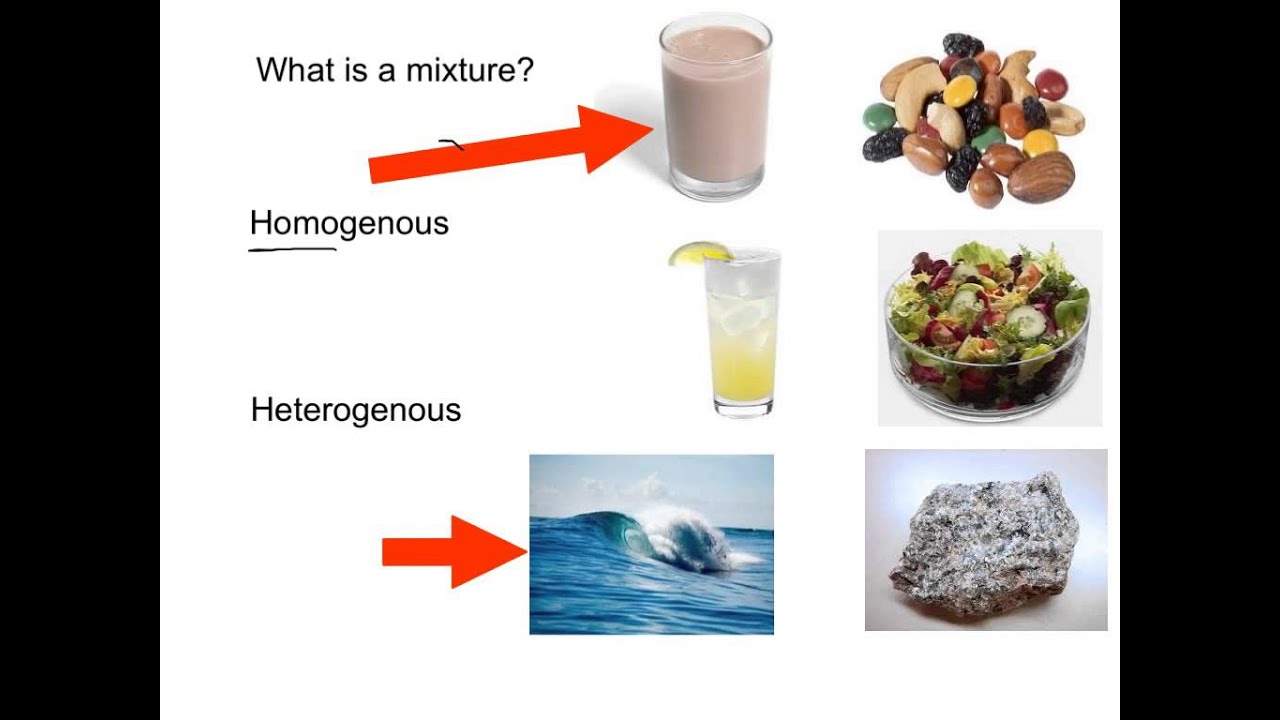



Mixtures Youtube



Elements Compounds And Mixtures Sas
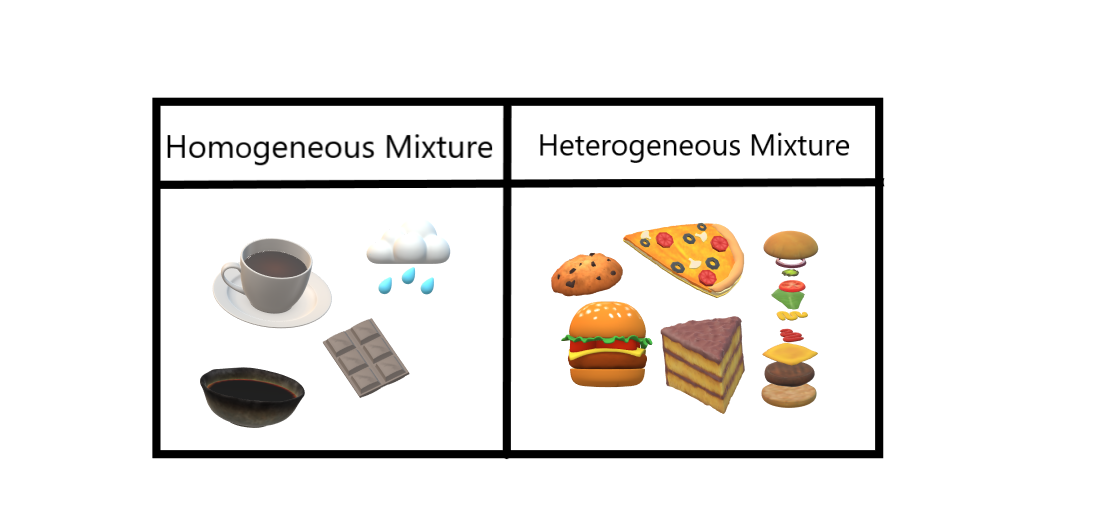



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube



Difference Between Homogenous And Heterogeneous Mixture Javatpoint



Is Sugar A Homogeneous Or Heterogeneous Mixture Chemistry For Neet




The Homogeneous And Heterogeneous Mixture Diagram Quizlet




Oneclass Classify Each Example As An Element A Compound A Heterogeneous Mixture Or A Homogeneous




Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture




Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool




What Is A Homogeneous Mixture Definition And Examples




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Properties Of Homogeneous Mixture The Difference Between Heterogeneous And Homogeneous Mixtures
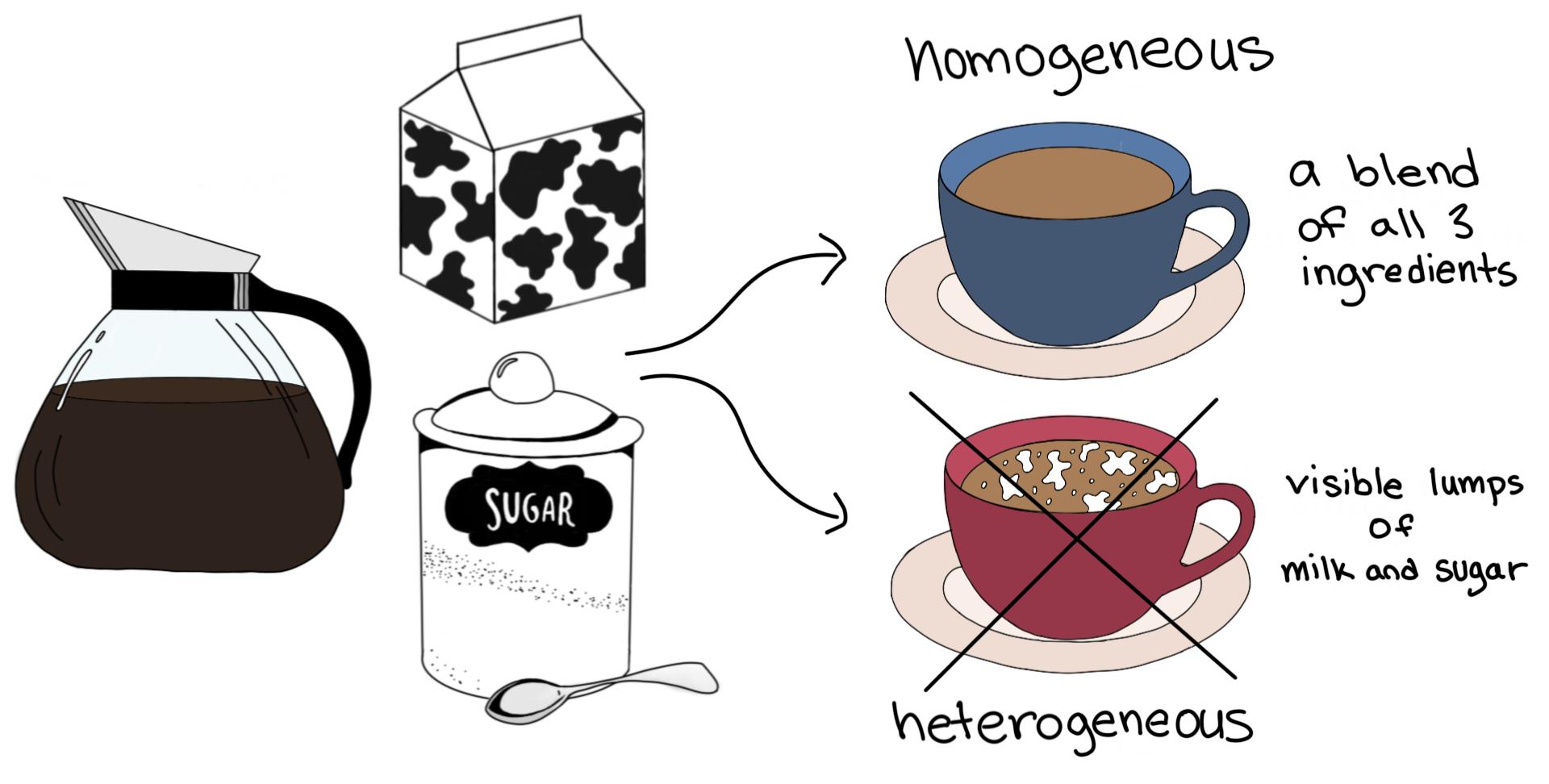



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
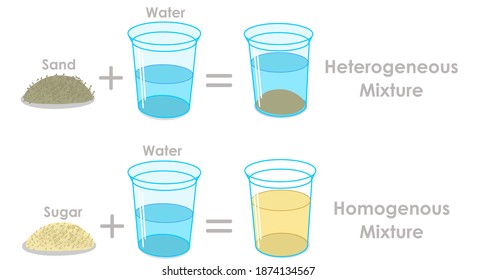



Mixtures Images Stock Photos Vectors Shutterstock




Mixtures Homogeneous And Heterogeneous Mixtures Ppt Video Online Download
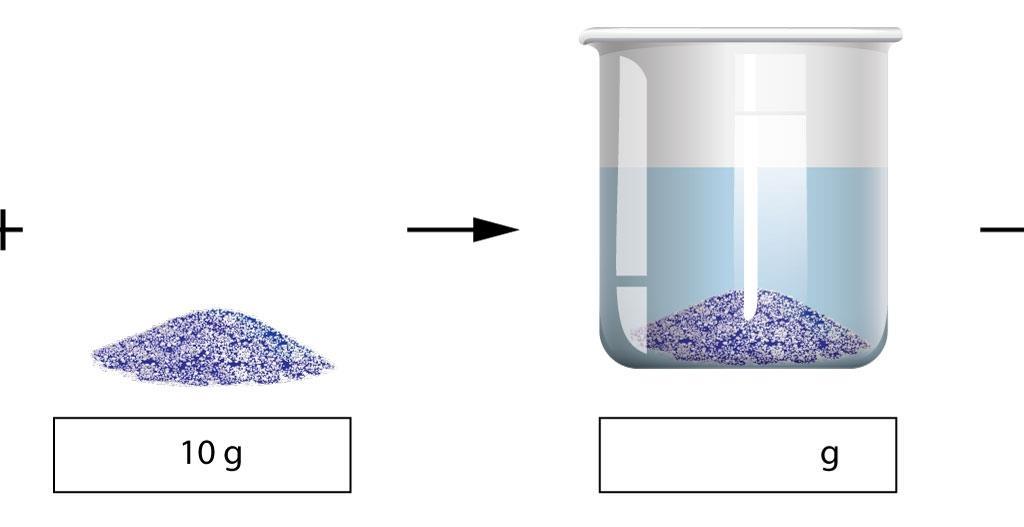



Mixtures And Solutions Cpd Rsc Education




G 3 In This 3 In This Experiment You Worked With Chegg Com



What Is A Gaseous Example Of A Heterogeneous Mixture Quora



3



Is Sugar A Homogeneous Or Heterogeneous Mixture Quora
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Ch2 Lesson A Page 2 When Can A Mixture Be Treated As A Pure Substance




Heterogeneous Homogeneous Mixture Card Sort For Matter In Chemistry
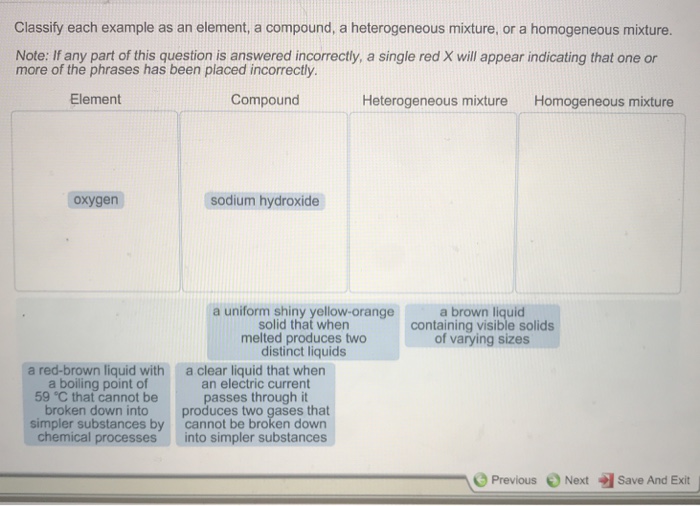



Classify Each Example As An Element A Compound A Chegg Com
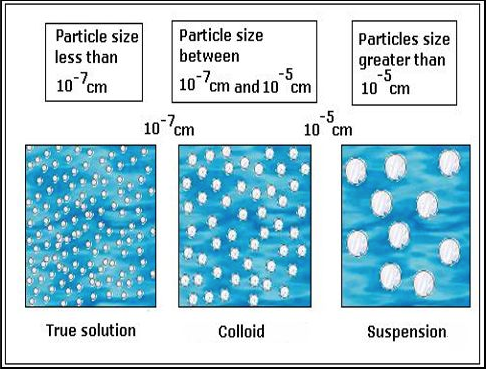



Are All Homogeneous Mixtures Also Solutions Example




Ch2 Lesson A Page 2 When Can A Mixture Be Treated As A Pure Substance



Homogeneous Vs Heterogeneous Matter Worksheet Answers Nidecmege




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube



1




Homogenous Definition And Examples Biology Online Dictionary
コメント
コメントを投稿